Research and Teaching
Medexter brings extensive expertise in clinical research and health IT education, using the ArdenSuite to develop prototypes, support grant proposals, and provide hands-on learning. The platform lets researchers and students explore real-world clinical decision support in a secure, standards-compliant environment.

The ArdenSuite in the Classroom
In academic settings, the ArdenSuite CDS platform offers students hands-on experience in real-world clinical decision support development. Universities have integrated the platform into master’s and doctoral programs in medical informatics, eHealth, and health services management, enabling learners to create their own Arden Syntax-based applications using the ArdenSuite’s editor tool.
With dedicated educational and research licenses, institutions can provide students and instructors access to the same technology used in healthcare environments. This allows future professionals to gain practical experience in building, testing, and deploying knowledge-based modules within a safe academic context.


The ArdenSuite in Research Projects
With its adaptable architecture, the ArdenSuite serves as a robust platform for cutting-edge clinical research.
Researchers integrate clinical decision support modules made with the ArdenSuite into studies to enhance trial design, data analysis, and outcomes. Several universities and hospitals have adopted the ArdenSuite and corresponding research & education licenses for projects spanning oncology, nephrology, dialysis, and dermatology. Compliant with interoperability standards such as FHIR, the platform supports the rapid development of custom CDS tools for research studies and grant proposals alike.
Whether creating proof-of-concept systems for personalized therapy or embedding alert modules into hospital workflows, the ArdenSuite provides a research-ready, standards-based framework.

Get your ArdenSuite license today.
License for Research & Education available.
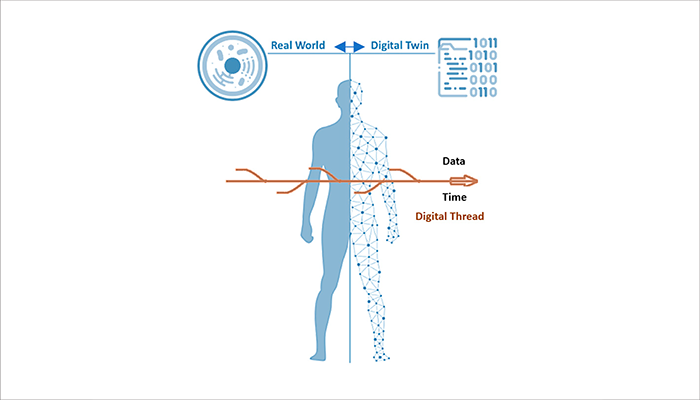
Health Digital Twins
Medexter has developed a prototype Health Digital Twin application that combines a three-dimensional patient avatar, advanced visualization of medical imaging data, and Arden Syntax–based clinical decision support.
Building on this foundation, ongoing development focuses on ensuring robust IT security and seamless integration with leading-edge EHR systems, in close collaboration with the Medical University of Vienna. Further details on the prototype are available in the following publications: https://doi.org/10.1007/978-3-031-63929-6_9

Digital Tumor Board
Digital precision medicine empowers tumor boards to make personalized cancer treatment decisions by analyzing genomic data from next-generation sequencing and drawing on prior outcomes to generate actionable reports. With proven expertise and validated software, Medexter is ready to support clinical decision-making in the field of precision oncology.


Moab – Monitoring of Antibiotics
Moab is a prototype electronic expert system designed to support empirical antibiotic therapy by leveraging clinical and resistance‑epidemiological data.
The system draws on a database of past microbiological cases, comparing the current patient’s infection profile and risk factors with similar historical records. Using fuzzy‑logic rules, Moab evaluates the case severity, pathogen spectrum, resistance patterns, comorbidities and contraindications, and then proposes a ranked list of antibiotic treatment options tailored to the patient.
Early testing in six realistic clinical scenarios showed that the system’s recommendations aligned with those published in the literature, demonstrating feasibility for decision support in empirical antibiotic management of urinary tract infections.


Made – Monitoring of Adverse Drug Events
Made is an intelligent monitoring system designed to enhance patient safety by detecting potential Adverse Drug Events (ADEs) in real time. Built with Arden Syntax MLMs, Made continuously analyzes clinical and lab data to identify early warning signs of drug-related complications, such as kidney issues, electrolyte imbalances, or over-anticoagulation. The system delivers smart alerts with risk scores, helping clinicians act quickly and accurately—without overwhelming them with false alarms.
In pilot studies, Made demonstrated strong sensitivity and reliability, proving its value in everyday hospital environments. By supporting timely intervention and streamlining ADE reporting, Made reduces underreporting, enhances pharmacovigilance, and ultimately improves outcomes. It’s a powerful tool for hospitals aiming to modernize their medication safety processes and support safer, smarter clinical decision-making.

Purchase your ArdenSuite license today.
Get your license or start a free trial.