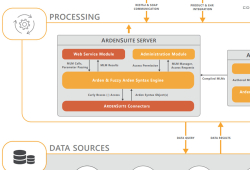
AMIA 2022 Annual Symposium, Washington, D.C.—Addressing the Curly Braces Problem
Medexter’s CEO Klaus-Peter Adlassnig co-authored a poster on “Implementing Health Level Seven Fast Healthcare Interoperability Resources (FHIR) as a Standard Data Model for the Arden Syntax.” Transferring medical logic modules (MLMs) from one site to another currently requires site specific…